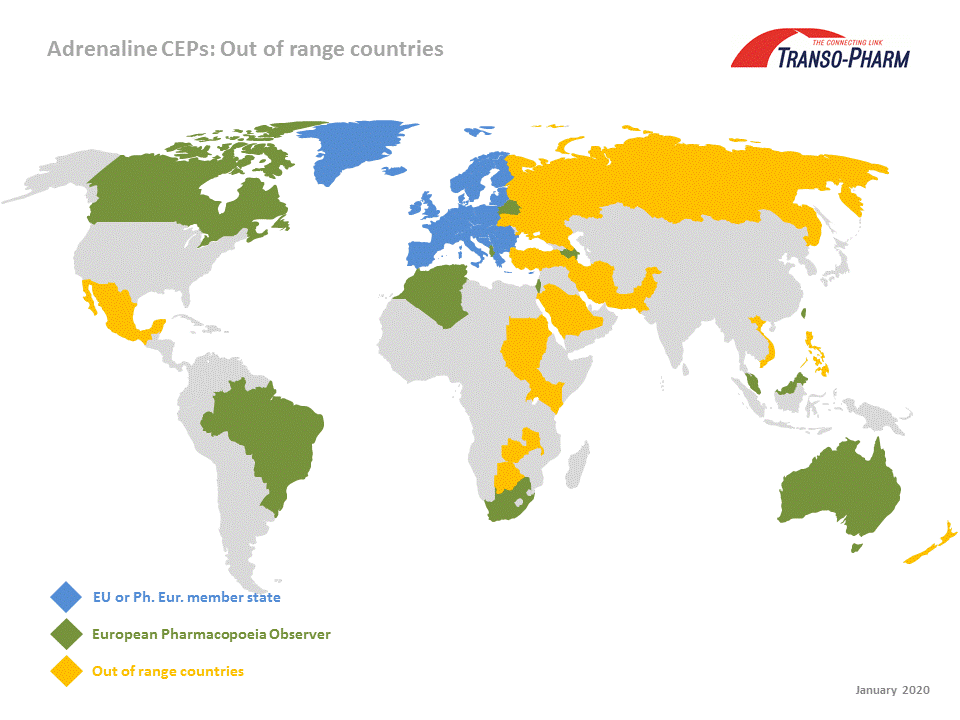
Adrenaline base and tartrate – Out of range CEP acceptance - our experience After successful process implementation for both Adrenalines/Epinephrines (based on Boehringer Ingelheims homogenous enantioselective hydrogenation technology) at our toll manufacturer Syn-Tech we obtained CEP 2016-232 for our Adrenaline and CEP 2016-233 for Adrenaline tartrate. API quality and registration documentation (CMC) corresponds with USDMF #30997 and #30996. Purpose and scope of a CEP is to surrogate the corresponding EDMF/ASMF as well as its individual review and assessment in each country in which the CMC is filed. As mentioned on EDQM´s homepage “Certificates of suitability (CEPs) are accepted in all EU member states and in member states of the Convention on the elaboration of a European Pharmacopoeia except Ukraine.” It goes without saying that we easily penetrated these markets utilizing our CEPs. But, what is about countries outside EU or Ph. Eur. member states? EDQM mentions “Based on information received from regulatory authorities and trade associations, the following countries accept CEPs, some with conditions: Albania, Algeria, Australia, Azerbaijan, Canada, Georgia, Ghana Israel, Kyrgyzstan, Malaysia, Moldova, Morocco, New Zealand, Saudi Arabia, Singapore, South Africa, Tunisia, and Uzbekistan. CEPs are also accepted (with conditions) by the Taiwan Food and Drug Administration.” Some of these have the European Pharmacopoeia Observer status. Meanwhile we adjusted our filing strategy in this matter and our Adrenaline CEPs have been submitted to several “out of range” countries. Country list contains
Some of those competent authorities asked for additional documents and/or information e. g. Attestation letter, CEP-dossier excerpt, commitment letter etc. Our experience is that more and more competent authorities outside EU and Ph. Eur. membership are willing to accept our Adrenaline CEPs, some with additional information. This encourages us to further expand the “out of range country list” and to apprise you, accordingly.
0 Comments
Your comment will be posted after it is approved.
Leave a Reply. |
Categories
All
Archives
June 2024
|
||||||||
ServicesProducts |
Company |
|

 RSS Feed
RSS Feed

