0 Comments
Last year we started with “We are going through some difficult times…”. Unfortunately we have to face the truth and state: “it ain’t over yet”. We all need a good portion of optimism for next year and should never give up hope.
With many thanks for your trust in us, we wish you, your family and friends a peaceful and happy Christmas and a healthy start into the New Year 2023. The first “nearly normal” CPHI after the strict pandemic rules took place in Frankfurt, Germany in November 2022. Despite the fact that the pharma community embraced the opportunity to see their partners face-to-face again or for the first time, it was not quite as crowded as in 2019. Chinas strict Covid policy and Visa issues especially in India prevented many visitors and even a couple of our partners from coming. We are all facing challenging times, but it seems that our partners, visitors and exhibitors were very happy to attend a real show with real people again. Talking, discussing and negotiating with peaceful humans from across the globe made us forget the global unrest for three days. Looking at so many happy faces during our Happy Hour made it clear that video conferencing will never replace the “real thing”. Transo-Pharm visited Crete, Greece in September 2022. It is the 6th major company trip that Transo-Pharm organized for us employees. Since 2014 I am working for Transo-Pharm and would not want to miss these highlights in my professional life. Some people might wonder why you would want to spend your private time with your colleagues as well, but if the working atmosphere is good and you have a lot of fun together, this question does not arise at all. We all enjoyed meeting our associates from Transo-Pharm USA, eating, chatting, laughing together and getting a good dose of greek sunshine. Thanks to the team who works with such dedication every day to ensure that our management can continue to make trips like this possible for us. Sincerely yours Maria Argiriou
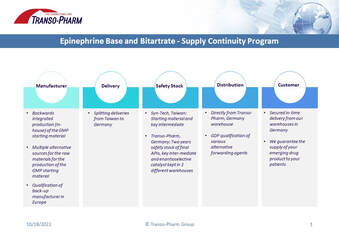
HAPILA and their distribution partner Transo-Pharm Handels GmbH have joined forces years ago in order to provide Estriol to more and more customers with high quality Estriol made in Germany. The collaboration entered into a new phase when we decided to expand capacity at the Hapila site in order to adequately supply the Estriol market. Consequently we made the decision to join forces at the CPhI Exhibition in Frankfurt and to integrate Hapila into the Transo-Pharm booth. A few recent updates on Estriol:
Looking forward to see you at CPHI in Frankfurt.  Friday evening – finally. Weekend is near...the time to relax and recharge the batteries. Here at Transo-Pharm we use this opportunity to meet with colleagues for a drink before heading off for the weekend. We don’t head to the bar, we gather in the office – under the clock. Special guests visiting us are always welcome to join as well. It is a nice ritual to get the team together after a long work week and a great opportunity to recap the weeks highlights and to exchange thoughts, ideas and information. Success stories are told as well as frustrating events are voiced. There are no filters; positive and negative emotions are let out and digested within the group. Of course, team discipline is key to this as well, as we all have to limit ourselves to one beer in order to be able to drive home safely and legally…and, of course, for those preferring something else to drink, we have a large range of non-alcoholic beverages available as well. Some very good and constructive ideas were born here. But the main thing is that we all feel as part of a ONE team…and that is a good feeling, based on the motto: “the best drinks are the ones you have with friends”. Friday after work at Transo-Pharm is our way to celebrate among co-workers and friends the end of a working week – no matter what…
|
Categories
All
Archives
March 2024
|
ServicesProducts |
Company |
|






 RSS Feed
RSS Feed

